Methotrexate induced neurotoxicity and reproductive dysfunction: role of honey and bee venom in modulating acetylcholine esterase activity, reproductive parameters and histopathological damage in albino rats
Bhalchandra Baburao Waykar , Dhanya Shrijith Pillai(Corresponding Author)
Department of zoology, babasaheb ambedkar marathwada university, chhatrapati sambhajinagar- 431004, Maharashtra, india
Doi -https://doi.org/10.1016/j.zool.2025.126572
ISSN - 0944-2006
Publication Date - August 22, 2025
Abstract
Objective:
Methotrexate (MTX) is a widely used chemotherapeutic and immunosuppressive agent associated with adverse effects such as neurotoxicity and reproductive dysfunction. This study aimed to evaluate the protective effects of honey and bee venom on MTX-induced toxicity in male albino rats.
Methods:
Thirty adult male rats were randomly assigned into five groups (n = 6 per group): normal control, MTX-treated, MTX + raw honey-treated, MTX + bee venom-treated, and MTX + raw honey + bee venom-treated. Raw Honey (1.2 g/kg, orally) and bee venom (0.5 mg/kg, intraperitoneally) were administered daily for 42 days, while MTX (5 mg/kg, intraperitoneally) was administered weekly once for 6 weeks. Evaluations included absolute and relative organ weights (brain and testis), sperm parameters (count, motility, viability), brain acetylcholinesterase (AChE) activity, and histopathological analysis of brain and testicular tissues.
Results:
MTX administration significantly decreased organ weights and reproductive indices, and increased AChE activity and tissue damage (p < 0.0001). Treatment with honey and bee venom ameliorated these effects, with the combination therapy showing the most pronounced improvements across all parameters. Histopathological assessments confirmed the restoration of normal architecture in brain and testicular tissues in the treated groups.
Conclusion:
Honey, especially when combined with bee venom, demonstrated antioxidant, anti-inflammatory, and neuroprotective properties that mitigated MTX-induced toxicity. These findings support the potential use of natural bioactive compounds as adjuvants to reduce the systemic side effects of chemotherapeutic agents like MTX.
Keywords: Methotrexate, Bee Venom, Honey, Neurotoxicity, Reproductive Toxicity
1. Introduction
‘Methotrexate’ (MTX) is a folate antagonist used by medical professionals to treat cancers and autoimmune conditions and inflammatory diseases such as psoriasis and rheumatoid arthritis (National Cancer Institute, 2024). However, MTX treatment has substantial neurotoxic and reproductive side effects, and these become worse with higher dose administration. Medical studies in pediatric oncology practice have demonstrated that MTX therapy causes stroke like neurotoxicity and seizures, leukoencephalopathy and cognitive impairments (Santangelo et al., 2022; Mateos et al., 2021; Shuper et al., 2000). According to Santangelo et al. (2022) and Shuper et al. (2000), MTX induced neurotoxicity is mediated by multiple physiological pathways including oxidative stress, excitotoxicity and inflammation, mitochondrial dysfunction and demyelination. Reproductive dysfunction is seen in patients who use MTX for long duration, which causes sperm degeneration and testicular atrophy and hormonal imbalance (Khalil et al., 2016). MTX has toxic side effects that reduce its therapeutic effectiveness, so researchers need to develop additional protective strategies that keep anticancer properties. The essential biomolecular target of MTX is acetylcholine esterase (AChE) which is the enzyme that breaks down acetylcholine and is responsible for creating neural damage. Abnormal AChE activity is a valuable biomarker for neurotoxicity because it is linked to synaptic transmission problems and memory and cognitive processing deficits (Mateos et al., 2021). The combination of therapeutic agents that control AChE activity with enhancement of an antioxidative defense system may be protective against MTX induced neural damage. Recently, natural compounds with the potential to reduce chemotherapy related toxicities have been of interest in the medical field. Bee venom (BV) and honey have been identified as prominent candidates for their protective properties that include both neuroprotection and antioxidant responses as well as anti-inflammatory functions. Experimental data indicates that bee venom with its main peptides melittin and apamin have positive effects in neurodegeneration and Parkinson’s disease research by decreasing neuronal cell death and improving motor skills (Badawi et al., 2020; Nguyen et al., 2022). BV activates ‘Nrf2/HO-1 and TrkB/CREB/BDNF pathways’ to achieve its cellular protective outcomes, which are able to reduce oxidative damage while maintaining neuronal survival (Nguyen et al., 2022). BV has protective effects that are beneficial to reproductive health functions. Several research investigations have demonstrated that BV enhances sperm quality by maintaining hormonal equilibrium and preserving testicular tissue structures through anti-inflammatory as well as antioxidative mechanisms (Suleiman et al., 2021; Aly et al., 2023). Stela et al. (2024) conducted research on a wide range of BV therapeutic uses and methods to improve its security and clinical performance. Clinical application of BV in neurological condition treatment by acupoint injections (Lin & Hsieh, 2020) reveals the therapeutic value of BV. Honey is a bee derived compound that contains flavonoids and phenolic acids along with enzymatic antioxidants. Honey administration has been shown to protect against MTX induced damage in brain and liver tissues as documented in lab research (Khalil et al., 2016). Honey is an attractive option as an additional therapy when receiving chemotherapeutic treatments because of its ability to regenerate tissues in neural and reproductive areas.
Despite rising interest, the combined application of honey and bee venom for MTX induced neurotoxicity and reproductive dysfunction protection has not been studied. Information on how these agents affect AChE activity and oxidative stress biomarkers, as well as the histopathological status of neural and reproductive tissues, has not been found in research. Additional research about improving quality of life through strategies that reduce adverse treatment effects brings us closer to the development of combined therapeutic approaches.
Objectives
The research examined how honey and bee venom protect the nervous system and reproductive function in albino rats exposed to methotrexate. Specifically, it aimed to:
-
To assess the effects of methotrexate on brain and testis weights and determine the protective role of honey and bee venom in preserving organ integrity.
-
To evaluate reproductive function through sperm count, motility, viability, and mortality, and examine the extent to which honey and bee venom reverse methotrexate-induced impairments.
-
To measure brain acetylcholinesterase (AChE) activity as an indicator of neurotoxicity and analyze the modulatory impact of honey and bee venom on enzymatic balance.
-
To investigate histopathological alterations in brain and testicular tissues following methotrexate exposure and assess tissue-level protection offered by honey and bee venom treatments.
The research explains protective mechanisms of natural substances which aims to foster integrated treatment methods against chemotherapeutic toxicity.
Materials and Methods
2.1. Ethical Compliance and Animal Maintenance
The research adhered to standards set by the Committee for the Control and Supervision of Experiments on Animals (CCSEA) through the Government of India as described in the Gazette of India Extraordinary (Part III) No. 270 dated December 15, 1998. The Institutional Animal Ethics Committee of Crystal Biological Solutions granted experimental permission and approval for this study under CRY/2425/182 (16/09/2024). Thirty albino healthy adult male rats served as experimental subjects in this study which obtained their source from animal house of Crystal Biologicals and received standard rat chow and water ad libitum while being housed in polypropylene cages under controlled environmental conditions of temperature at 22 ± 2°C, relative humidity at 55 ± 5% and 12 hours of light/dark cycle.
2.2. Experimental Design and Grouping
Rats were randomized into five groups (n = 6 per group). Methotrexate (MTX) was used to induce neurotoxicity and reproductive dysfunction. Treatment regimens included honey and bee venom, administered individually or in combination.
Table 1. Experimental Groups and Dosage Regimens
Methotrexate-Induced Toxicity Protocol
Methotrexate (MTX), a commonly prescribed agent for cancer and autoimmune disorders, is well-documented for inducing organ toxicity through mechanisms involving oxidative stress, inflammatory responses, and cellular damage. In the current study, experimental rats were exposed to MTX at a dose of 5 mg/kg body weight via intraperitoneal injection once per week for a duration of 42 days to establish a toxicity model.
-
This dosage and schedule were adopted based on previously published findings that reported significant liver, kidney, and reproductive system damage following MTX administration. ( Cianciosi et al.,2018; Ghoneum et al.,2021;Ahmed et al.,2021)
-
To explore potential protective interventions:
Bee venom was administered daily at 0.5 mg/kg body weight intraperitoneally Meligi et al.,2020; Zahran et al.,2021)
Honey was given orally at 1.2 g/kg body weight per day (Alturkistani et al., 2019;Sadek et al.,2024)
2.3. Determination of Organ Weights
Rats were anesthetized and euthanized after post-treatment. Absolute and relative organ weights were determined by excising brain and testis tissues, washing in saline, and weighing. The relative organ weight was calculated by:
2.4. Evaluation of Reproductive Parameters
These tissues samples were followed up with excision of the cauda epididymis and placing it in 1.0 ml of PBS solution with a pH of 7.4. It was then minced into smaller samples with the aid of sharp surgical scissors and scalpel for better representation and accuracy. This suspension was then allowed to stand at the temperature of 37 degree Celsius for a period of ten minutes.
• Quantified sperm count using a Neubauer hemocytometer.
• Microscopic observation of sperm motility and expressed as a percentage.
• Assessment of Sperm Viability: Live and dead spermatozoa were distinguished using eosin nigrosin staining.
2.5. Assessment of Acetylcholinesterase (AChE) Activity
According to the manufacturer’s instructions, protein extraction of homogenized brain tissues was done using the commercially available ELISA kits.
ELISA Procedure
Each well received 50 µL of standard solution during the ELISA procedure. The procedure required the addition of 100 µL test samples or standard diluent into their corresponding wells. The plates underwent 80-minute incubation at 37°C before the plates received four washes. The procedure continued with streptavidin enzyme conjugate addition to each well followed by a 37°C incubation period of 30 minutes before adding biotinylated acetylcholinesterase antibody (AChE) to each well for 50 minutes at 37°C. The next washing procedure was followed by Streptavidin – HRP and TMB substrate addition of 100 µL each to all wells. The reaction required 10 minutes at 37°C until the stop solution (100 µL) was added to terminate the reaction. The microplate reader measured absorbance at wavelength 450 nm as the final step.
2.6. Histopathological Examination
Cell tissue preservation occurred through formalin fixation of brain and testis tissues prior to sectioning at 5 microns. Subsequent staining with hematoxylin and eosin took place for both specimens. The light microscope at 40X magnification was used to observe histoarchitectural changes in the sections.
2.7. Statistical Analysis
The researchers presented categorical experimental data as mean ± Standard Deviation (SD) values through software GraphPad Prism for their calculations. The following procedure was used to analyse between group differences: The Student’s t-test analysed the data between normal and disease control groups. Dunnett’s post hoc test served to analyse the multiple group comparisons against the disease control group.
The current study established P < 0.05 as its significant level.
3. Results
3.1. Effect on Absolute and Relative Organ Weights
They found out that the average organ coefficients of the brains and testes in the methotrexate administered group were significantly lower than those of the normal control group with p < 0.01, p < 0.001 and p < 0.0001 respectively. Honey, bee venom or a combination of the two protected against the following damage: a significant restoration of organ weights, the honey produced the best protective response and the honey combined with bee venom produced the best combined protective response.
(‘Values are expressed as mean ± SD. Number of animals in all experimental groups (n) = 6. Significant differences were observed as*p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001. aNormal Control Vs. Disease control group was analyzed by Student’s t test. bDisease Control Vs. Treatment groups were analysed by One-way ANOVA followed by Dunnet’s comparison test’)
3.2. Effect on Reproductive Parameters
Methotrexate significantly reduced sperm count, motility, and live sperm percentage, while increasing dead sperm levels (p < 0.0001). Treatment with honey, bee venom, and especially their combination resulted in significant recovery in all parameters (p < 0.0001), with the combination group achieving results closest to normal values.
Table 5. Effect on Reproductive Parameters
(‘Values are expressed as mean ± SD. Number of animals in all experimental groups (n) = 6. Significant differences were observed as*p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001. aNormal Control Vs. Disease control group was analyzed by Student’s t test. bDisease Control Vs. Treatment groups were analysed by One-way ANOVA followed by Dunnet’s comparison test’)
Figure 1: Bar graphs showing sperm count (A), motility (B), live (C) and dead sperm percentage (D)
(‘Values are expressed as mean ± SD. Number of animals in all experimental groups (n) = 6. Significant differences were observed as*p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001. Normal Control Vs. Disease control group was analyzed by Student’s t test. Values of Disease Control Vs. Treatment groups were analyzed by One-way ANOVA followed by Dunnet’s comparison test’)
3.3. Effect on Acetylcholinesterase (AChE) Activity in Brain Tissue
In the current study, MTX led to a significant increase in brain AChE activity when determined from the data; F (3, 53) = 17.51, p < 0.0001. However, all honey treatment, bee venom treatment, and combined treatment demonstrated significant reduction on AChE level than the disease group (p < 0.0001) and the combined treatment nearly approached the normal control level.
3.4. Histopathological Evaluation of Testes
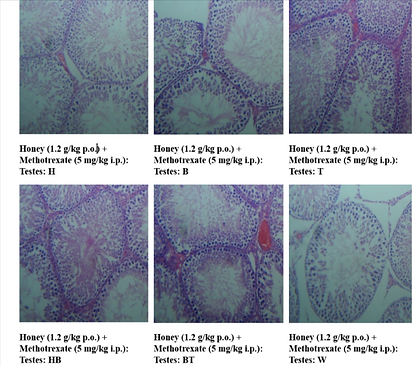
Histological analysis of testis tissue from the methotrexate-treated group showed marked atrophy, degeneration of seminiferous tubules, and decreased spermatogenesis. Testes from rats treated with honey, bee venom, or both exhibited preserved architecture and restored spermatogenic activity, with the combination group displaying the closest resemblance to the control. Here H,B,T,HB,BT,W are the animal identification markings which stands for H-Head, B-Back, T-Tail, HB-Head Back, BT-Back Tail, W-White.
Figure 3(a), (b): Photomicrographs of testis tissue – (a) Normal Control and (b) Disease Control
Figure 3(a)Normal Control Group: Showing normal seminiferous tubules, spermatocytes and germinal cells. 40X, H & E Stain.
Figure 3(b)Disease Control: Methotrexate 5 mg/kg i.p. Group Showing Atrophy and degeneration of seminiferous tubules, multifocal. 40X, H & E Stain.
Figure 4: Photomicrographs of testis tissue – Honey, Bee Venom, and Combination treatment groups
Figure 4(a):Honey (1.2 g/kg p.o.) + Methotrexate (5 mg/kg i.p.) Group Showing normal seminiferous tubules, spermatocytes and germinal cells. 40X, H & E Stain.
3.5. Histopathological Evaluation of Brain
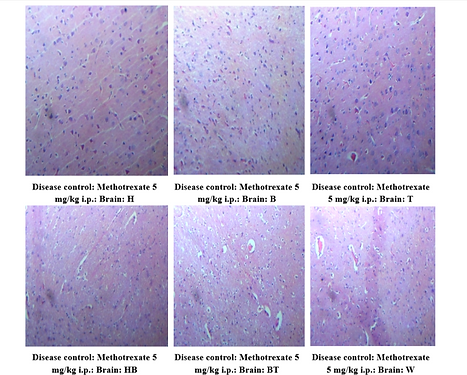
Brain sections from the MTX group demonstrated multifocal necrosis and degeneration in the cerebral parenchyma. These changes were ameliorated in all treated groups, with near-normal histological features observed in the combination therapy group. Here H,B,T,HB,BT,W are the animal identification markings which stands for H-Head, B-Back, T-Tail, HB-Head Back, BT-Back Tail, W-White)
Figure 5: Brain histopathology – Normal (a) and Disease Control groups (b)
Figure 5(a): Normal Control Group Showing normal cerebral parenchyma. 40X, H & E Stain.
Figure 5(b): Disease Control: Methotrexate 5 mg/kg i.p. Group Showing Neuronal necrosis in cerebral parenchyma, multifocal. 40X, H & E Stain.
Figure 6: Brain histopathology – Honey (a), Bee Venom(b), and Combination therapy groups (c)
Figure 6(a): Honey (1.2 g/kg p.o.) + Methotrexate (5 mg/kg i.p.) Group Showing normal cerebral parenchyma. 40X, H & E Stain.
Figure 6 (b): Bee Venom (0.5 mg/kg i.p.) + Methotrexate (5 mg/kg i.p.) Group Showing normal cerebral parenchyma. 40X, H & E Stain
Figure 6(c): Honey (1.2 g/kg p.o.) + Bee Venom (0.5 mg/kg i.p.) + Methotrexate (5 mg/kg i.p.) Group Showing normal cerebral parenchyma. 40X, H & E Stain.
4. Discussion
The research shows that the chemotherapeutic agent methotrexate (MTX) produces substantial neurotoxic and reproductive damage in male albino rats through its effects on brain weight reduction and elevated acetylcholinesterase (AChE) activity and sperm parameter deterioration and brain and testicular tissue histopathological damage. The observed MTX-induced outcomes demonstrate how this drug damages both neural and reproductive systems through its established mechanisms of oxidative stress and inflammation and apoptosis. The MTX-treated group showed notable AChE activity elevation while under examination which demonstrated cholinergic dysfunction alongside neuronal stress. Research evidence shows that elevated AChE activity serves as a key marker for neurotoxicity which develops from oxidative stress and synaptic breakdown. The normalization of AChE levels through honey and bee venom therapy worked best when administered together to reestablish cholinergic function plus synaptic homeostasis. Szwajgier et al. (2022) demonstrated that different honeys function as natural cholinesterase inhibitors according to their research. The research established that particular honey varieties decrease AChE activity which leads to better stability of cognitive functions and neurophysiological processes. The neuroprotective actions of honey were explained mechanistically by Dar (2020) through his discussion of flavonoid content and free radical scavenging and anti-inflammatory properties. The mechanisms described explain how honey treatment led to normal AChE activity levels and better tissue organization in our rat study. The neurotoxicity-modulating properties of bee venom became evident through its strong ability to decrease AChE activity and improve the integrity of neuronal tissue. The research of Alparslan et al. (2024) showed that bee venom and its main component melittin function as strong enzyme-blocking substances which demonstrate substantial neuroprotective properties. The study confirmed that melittin blocked AChE activity while activating anti-inflammatory pathways which led to cholinergic disturbance reversal during this experiment. The Nrf2/HO-1 pathway and other antioxidant cascades become activated by melittin as Nguyen and Lee (2021) showed in their research while supporting neuroprotection against oxidative stress-induced neurodegeneration which corresponded to our study results of biological recovery and histopathological improvements in combination therapy application. The MTX group exhibited brain necrosis and degeneration throughout multiple brain regions but the brain tissue of treatment groups including honey and bee venom combination therapy showed preserved brain structures. The research by Aly et al. (2023) showed that bee venom effectively decreased hippocampal oxidative damage and neuroinflammation during epileptic brain insults. Results in our research mirror past findings about bee venom because this substance protects tissue structures by strengthening cell membranes and decreasing inflammatory response while promoting brain cell healing. The experimental research conducted by Memudu et al. (2024) showed how honey treated traumatic brain injuries by reducing anxiety levels and enhancing motor performance through its ability to manage oxidative stress effects on behavioral measures. Our findings regarding cognitive and structural improvements from honey treatment in MTX models are supported by the neurobehavioral results we obtained. Exposure to Methotrexate resulted in a major reduction of sperm count and motility and viability while producing testicular degeneration signs that match the established gonadotoxic effects of the drug. The reproductive damage caused by oxidative cell damage to spermatogenic cells and testicular microarchitecture disruption occurs commonly in such cases. Both single application of honey and bee venom therapy regenerated sperm cell health and repaired testicular structures but the combinations resulted in superior repair outcomes. Ghafouri-Fard et al. (2021) reviewed how chemotherapeutic agents affect male germ cells while showing that antioxidants protect fertility by stopping DNA fragmentation and apoptosis. Kurt et al. (2020) showed that vitamin E antioxidants effectively decrease methotrexate-induced testicular damage and apoptosis which supports the mechanism behind the natural agents we studied. The research by Ibrahim et al. (2016) showed that honey combined with royal jelly effectively reduced cisplatin-induced renal and systemic toxicity because of their anti-inflammatory and antioxidant properties. Our research confirms the results by showing bee-derived substances have a protective effect that extends to reproductive toxicity from methotrexate exposure. Combined treatment of honey and bee venom demonstrated the best results in restoring normal physiological, biochemical and histological parameters. The two agents interact synergistically because honey supplies antioxidants while bee venom activates apamin and melittin peptide defense mechanisms against inflammation. According to Zhang et al. (2018) the synergistic bioactivity between bee venom and honey has been extensively supported through their review of therapeutic mechanisms and potential applications.
5. Conclusion
A cross-sectional study on the effects of methotrexate demonstrates that the drug possesses neurotoxic and reproductive toxicity resulting from systemic administration. Collectively, the significant decline in absolute and relative organ weights, impairment in quality and viability of sperm with elevation of brain acetylcholinesterase (AChE) activity and histopathological alterations in brain and testicular tissues resulting due to MTX are indicative of its remarkable physiological disruption. The effects observed are in accordance with the known pathophysiological mechanisms of MTX including oxidative stress, inflammatory response and disturbance of cellular homeostasis. Importantly the administration of honey and bee venom both known natural therapeutic agents provided significant protection against MTX induced damage. Honey itself is rich in flavonoids, phenolic compounds and the antioxidant enzymes that act to counter oxidative stress and support tissue regeneration. Traditional bioactive peptides from bee venom such as melittin and apamin can cause mediated anti-inflammatory as well as immunomodulatory properties that can help maintain the performance integrity of neuronal and reproductive tissues respectively. Together, these agents had a synergistic effect, to restore organ function and morphology better than when used alone. The hitting of all the parameters appeared particularly encouraging in the combined treatment group, where nearly all parameters approached normal levels. The AChE activity was reduced in treated groups, this indicates that not only do these agents diminish neurotoxicity but also maintain both cognitive and synaptic reserve. Also, the increase in sperm count, motility and viability shows that honey and bee venom affect testicular antioxidant systems, maintain germinal epithelium and improve spermatogenesis. These biochemical and physiological findings were reinforced by histopathological evaluations performed in treated animals that showed improved tissue integrity with architectural preservation in both the brain and testes. The evidenced based findings should therefore encourage the use of honey and particularly the bee venom for enhancing the negative impact of chemotherapy. This study’s overall outcomes indicate that honey and bee venom exhibits benefits in the recovery of neurotoxicity and revives the reproductive system impaired by MTX through the possible antioxidant, anti-inflammatory, and neuroprotective mechanisms. Future investigations to identify the molecular pathways involved and determine the translational potentials in clinics using these natural agents can be provided by these findings. Studies with further in depth analyses such as molecular profiling, dose optimization, long term toxicity evaluation and eventually clinical trials will be needed to establish their safety and efficacy in humans using methotrexate or other chemotherapeutic agents.
References
-
"Methotrexate." National Cancer Institute Drug Dictionary. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/methotrexate
-
Santangelo, A., Bartolini, E., Nuzzi, G., Foiadelli, T., Michev, A., Mina, T., ... & Orsini, A. (2022). The clinical impact of methotrexate-induced stroke-like neurotoxicity in paediatric departments: An Italian multi-centre case-series. Frontiers in Neurology, 13, 920214.
-
Mateos, M. K., Marshall, G. M., Barbaro, P. M., Quinn, M. C., George, C., Mayoh, C., & Trahair, T. N. (2021). Methotrexate-related central neurotoxicity: clinical characteristics, risk factors and genome-wide association study in children treated for acute lymphoblastic leukemia. Haematologica, 107(3), 635.
-
Shuper, A., Stark, B., Kornreich, L., Cohen, I. J., Aviner, S., Steinmetz, A., ... & Yaniv, I. (2000). Methotrexate treatment protocols and the central nervous system: significant cure with significant neurotoxicity. Journal of child neurology, 15(9), 573-580.
-
Khalil, F. A., EL-Kirsh, A. A., Kamel, E. A., & EL-Rahmany, N. G. (2016). Beneficial effect of propolis extract (bee glue) against methotrexate-induced stress in liver and brain of albino rats. Indian J. Med. Res. Pharm. Sci, 3, 24-35.
-
Badawi, H. M., Abdelsalam, R. M., Abdel-Salam, O. M., Youness, E. R., Shaffie, N. M., & Eldenshary, E. E. D. S. (2020). Bee venom attenuates neurodegeneration and motor impairment and modulates the response to L-dopa or rasagiline in a mice model of Parkinson’s disease. Iranian Journal of Basic Medical Sciences, 23(12), 1628.
-
Nguyen, C. D., Yoo, J., Hwang, S. Y., Cho, S. Y., Kim, M., Jang, H., & Lee, G. (2022). Bee Venom Activates the Nrf2/HO-1 and TrkB/CREB/BDNF Pathways in Neuronal Cell Responses against Oxidative Stress Induced by Aβ1–42. International Journal of Molecular Sciences, 23(3), 1193.
-
Suleiman, J. B., Bakar, A. B. A., & Mohamed, M. (2021). Review on bee products as potential protective and therapeutic agents in male reproductive impairment. Molecules, 26(11), 3421.
-
Aly, E. K., Mahmoud, H. S., Alkhalifah, D. H. M., Shehab, G. M., Abuelsaad, A. S., Abdel-Rehiem, E. S., & Abdul-Hamid, M. (2023). Bee venom ameliorates oxidative stress and histopathological changes of hippocampus, liver and testis during status epileptics. Neuropeptides, 101, 102368.
-
Stela, M., Cichon, N., Spławska, A., Szyposzynska, M., & Bijak, M. (2024). Therapeutic Potential and Mechanisms of Bee Venom Therapy: A Comprehensive Review of Apitoxin Applications and Safety Enhancement Strategies. Pharmaceuticals, 17(9), 1211.
-
Lin, T. Y., & Hsieh, C. L. (2020). Clinical applications of bee venom acupoint injection. Toxins, 12(10), 618.
-
Cianciosi, D., Forbes-Hernández, T. Y., Afrin, S., Gasparrini, M., Reboredo-Rodriguez, P., Manna, P. P., Zhang, J., Bravo Lamas, L., Martínez Flórez, S., Agudo Toyos, P., Quiles, J. L., Giampieri, F., & Battino, M. (2018). Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules (Basel, Switzerland), 23(9), 2322. https://doi.org/10.3390/molecules23092322
-
Ghoneum, M., & El-Gerbed, M. S. A. (2021). Human placental extract ameliorates methotrexate-induced hepatotoxicity in rats via regulating antioxidative and anti-inflammatory responses. Cancer chemotherapy and pharmacology, 88(6), 961–971. https://doi.org/10.1007/s00280-021-04349-4
-
Ahmed, Z. S. O., Hussein, S., Ghandour, R. A., Azouz, A. A., & El-Sakhawy, M. A. (2021). Evaluation of the effect of methotrexate on the hippocampus, cerebellum, liver, and kidneys of adult male albino rat: Histopathological, immunohistochemical and biochemical studies. Acta Histochemica, 123(2), 151682.
-
Meligi, N. M., Ismail, S. A., & Tawfik, N. S. (2020). Protective effects of honey and bee venom against lipopolysaccharide and carbon tetrachloride-induced hepatoxicity and lipid peroxidation in rats. Toxicology research, 9(5), 693–705. https://doi.org/10.1093/toxres/tfaa077
-
Zahran, F., Mohamed, A., & Zein, N. (2021). Bee venom attenuates degenerative effects of diabetes associated with hyperlipidemia in rats. Biochemistry Letters, 17(1), 77-107.
-
Alturkistani, H. A., Abuzinadah, O. A., Kelany, A. M., Aziz, G. S., & Alrafiah, A. R. (2019). The combined effect of honey and olive oil against methotrexate mediated hepatotoxicity in rats: A biochemical, histological and immunohistological study.
-
Sadek, K. M., Shib, N. A., Taher, E. S., Rashed, F., Shukry, M., Atia, G. A., Taymour, N., El-Nablaway, M., Ibrahim, A. M., Ramadan, M. M., Abdelkader, A., Abdo, M., Imbrea, I., Pet, E., Ali, L. S., & Abdeen, A. (2024). Harnessing the power of bee venom for therapeutic and regenerative medical applications: an updated review. Frontiers in pharmacology, 15, 1412245. https://doi.org/10.3389/fphar.2024.1412245
-
Szwajgier, D., Baranowska-Wójcik, E., Winiarska-Mieczan, A., & Gajowniczek-Ałasa, D. (2022). Honeys as possible sources of cholinesterase inhibitors. Nutrients, 14(14), 2969.
-
Dar, N. J. (2020). Neuroprotective Effects of Honey: A Mechanistic View. Therapeutic Applications of Honey and its Phytochemicals: Volume II, 45-60
-
Alparslan, B., Şentürk, M., & Erkan, C. (2024). Bee venom and melittin: Potent key enzyme inhibitors with promising therapeutic potential. Toxicon, 252, 108164.
-
Nguyen, C. D., & Lee, G. (2021). Neuroprotective activity of melittin—the main component of bee venom—Against oxidative stress induced by Aβ25–35 in in vitro and in vivo models. Antioxidants, 10(11), 1654.
-
Memudu, A. E., Dennis, D., & Chatterjee, I. (2024). Neuroprotective effects of honey against traumatic brain injury-induced anxiety and motor function impairment in Wistar rats. Neuroscience Research Notes, 7(4), 360-1Darwish, S. F., El-Bakly, W. M., Arafa, H. M., & El-Demerdash, E. (2013). Targeting TNF-α and NF-κB activation by bee venom: role in suppressing adjuvant induced arthritis and methotrexate hepatotoxicity in rats. PLoS One, 8(11), e79284.
-
Ghafouri-Fard, S., Shoorei, H., Abak, A., Seify, M., Mohaqiq, M., Keshmir, F., & Ayatollahi, S. A. (2021). Effects of chemotherapeutic agents on male germ cells and possible ameliorating impact of antioxidants. Biomedicine & Pharmacotherapy, 142, 112040.
-
Kurt, Ş., Erişgin, Z., Tekelioğlu, Y., Akman, A., & Türedi, S. (2020). Protective effects of vitamin E against methotrexate-induced testicular damage in rats: Histopathologic and flow cytometric study. Celal Bayar Üniversitesi Sağlık Bilimleri Enstitüsü Dergisi, 7(3), 278-284.
-
Ibrahim, A., Eldaim, M. A. A., & Abdel-Daim, M. M. (2016). Nephroprotective effect of bee honey and royal jelly against subchronic cisplatin toxicity in rats. Cytotechnology, 68(4), 1039-1048.
-
Zhang, S., Liu, Y., Ye, Y., Wang, X. R., Lin, L. T., Xiao, L. Y., & Liu, C. Z. (2018). Bee venom therapy: Potential mechanisms and therapeutic applications. Toxicon, 148, 64-73.